Water purification removes contaminants from water, including biological contaminants, suspended solids and gases.
Tap water contains numerous contaminants and even trace impurity levels, which, while fine to drink, can affect various scientific experiments. To get good, reliable, accurate scientific data from experiments, it is vital that you use purified water for making up buffers or rinsing glassware.
We at Fistreem International have designed economic water purification systems that guarantee pure water for all your laboratory needs.
Technologies
There are several different methods of water purification that you can use to create pure water. The technology best suited for your needs depends on the application you require, the grade of water needed and the level of impurities in your water.
Your Content Goes Here
Your Content Goes HereDistillation
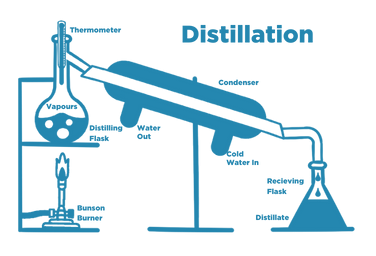
Distillation is a long-established method that separates water from contaminants by changing the state of water from a liquid phase to a gas phase and then back to a liquid phase.
Water is first heated to boiling, and water vapour rises to a condenser. Cooling water lowers the temperature, so the vapour is condensed, collected, and stored.
Distillation removes the following water impurities
- Organic compounds
- Inorganic salts
- Microrganisms and biomolecules
- Pyrogens
Your Content Goes Here
Your Content Goes HereReverse Osmosis (RO)
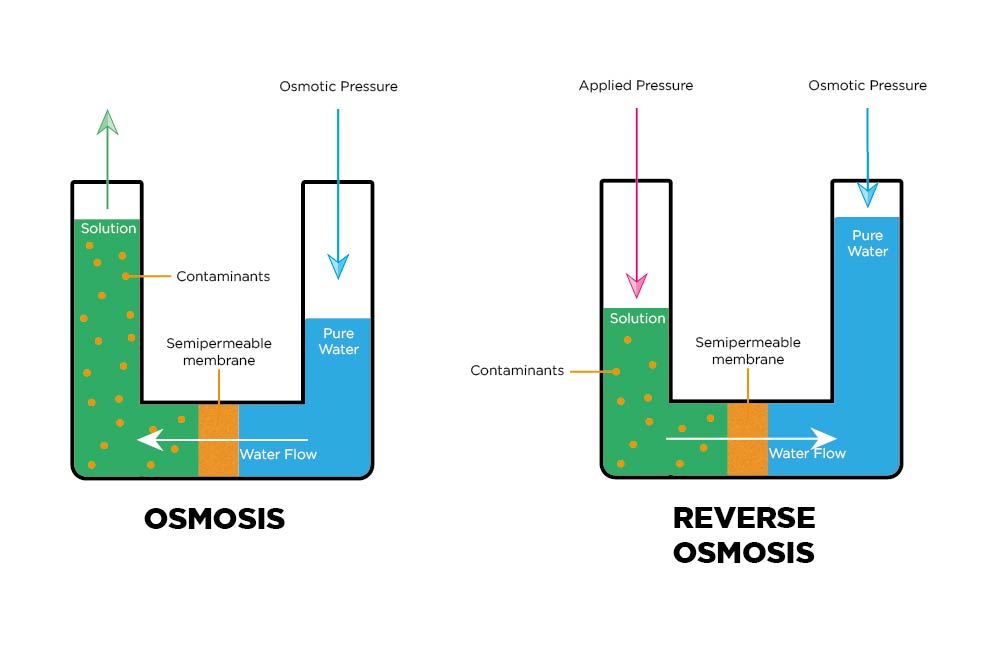
In the osmosis process, a solvent naturally moves from a low solute concentration (high water potential) through a membrane to a place of high solute concentration (low water potential).
Reverse osmosis, an applied pressure, is used to overcome osmotic pressure. The solute is retained on the membrane’s pressurised side, and the pure solvent can pass to the other side.
Reverse Osmosis removes the following water impurities
- Organic compounds
- Inorganic salts
- Microorganisms and Biomolecules
- Pyrogens
Your Content Goes Here
Your Content Goes HereDeionisation
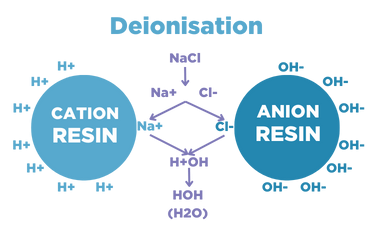
Deionisation uses synthetic ion-exchange resins to remove ions from feedwater chemically. As the water passes through, hydrogen and hydroxide ions exchange for dissolved minerals and then recombines to form water.
Deionisation resin beds or columns are typically made from cation-exchange and anion-exchange resins in separate beds or packaged together. There are three types of deionisation: co-current, counter-current and mixed bed.
Co-current deionisation refers to the original downflow process where input water and regeneration chemicals enter at the top of an ion exchange column and exit at the bottom.
Counter-current deionisation comes in two forms. The first form is up flow columns where input water enters from the bottom and regenerates from the top of the exchange column. The second form is up flow regeneration, where water enters from the top and restores from the bottom.
Mixed bed deionisation is a 50/50 mixture of carbon and anion resin combined in a single ion exchange column.
Deionisation removes the following water impurities:
- Inorganic ions
Your Content Goes Here
Your Content Goes HereUltra-Violet (UV)
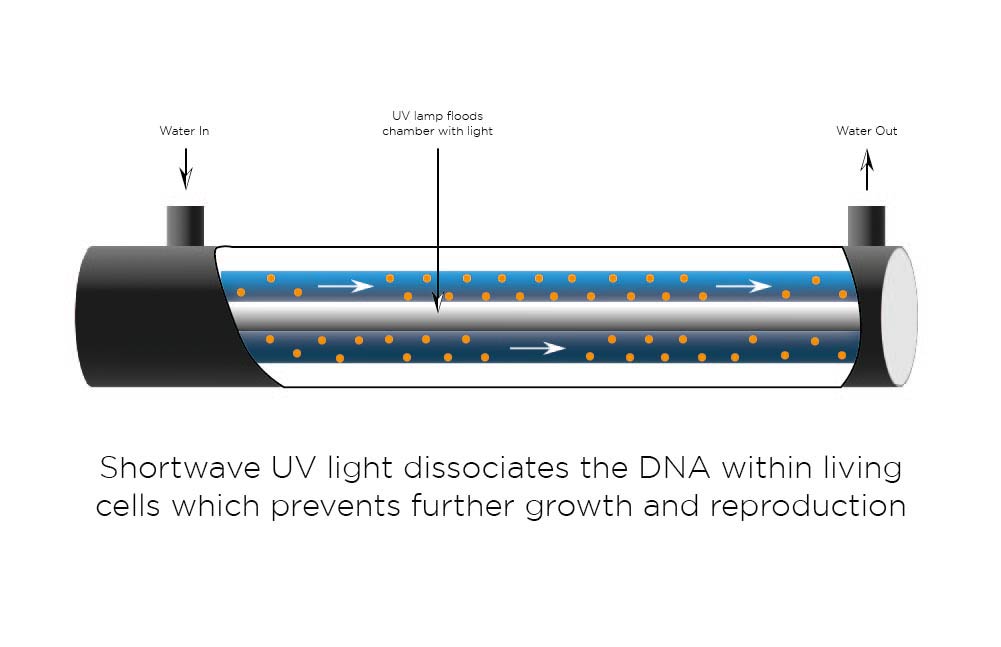
UV is used to disinfect water. Two wavelengths are commonly used, 185nm and 254nm.
254nm UV has a robust bacterial action as it damages DNA and RNA polymerase at low doses preventing replication. UV light at 185nm has a strong oxidising action which breaks down large organic molecules into smaller ionised components, ultimately CO2.
A UV chamber typically consists of a UV lamp mounted in a quartz tube in the centre of a stainless-steel tube. Water then flows through the zone between the quartz and the steel tubes.
Ultra-Violet removes the following water impurities:
- Trace organic compounds
- Inactivates micro-organisms and biomolecules
